
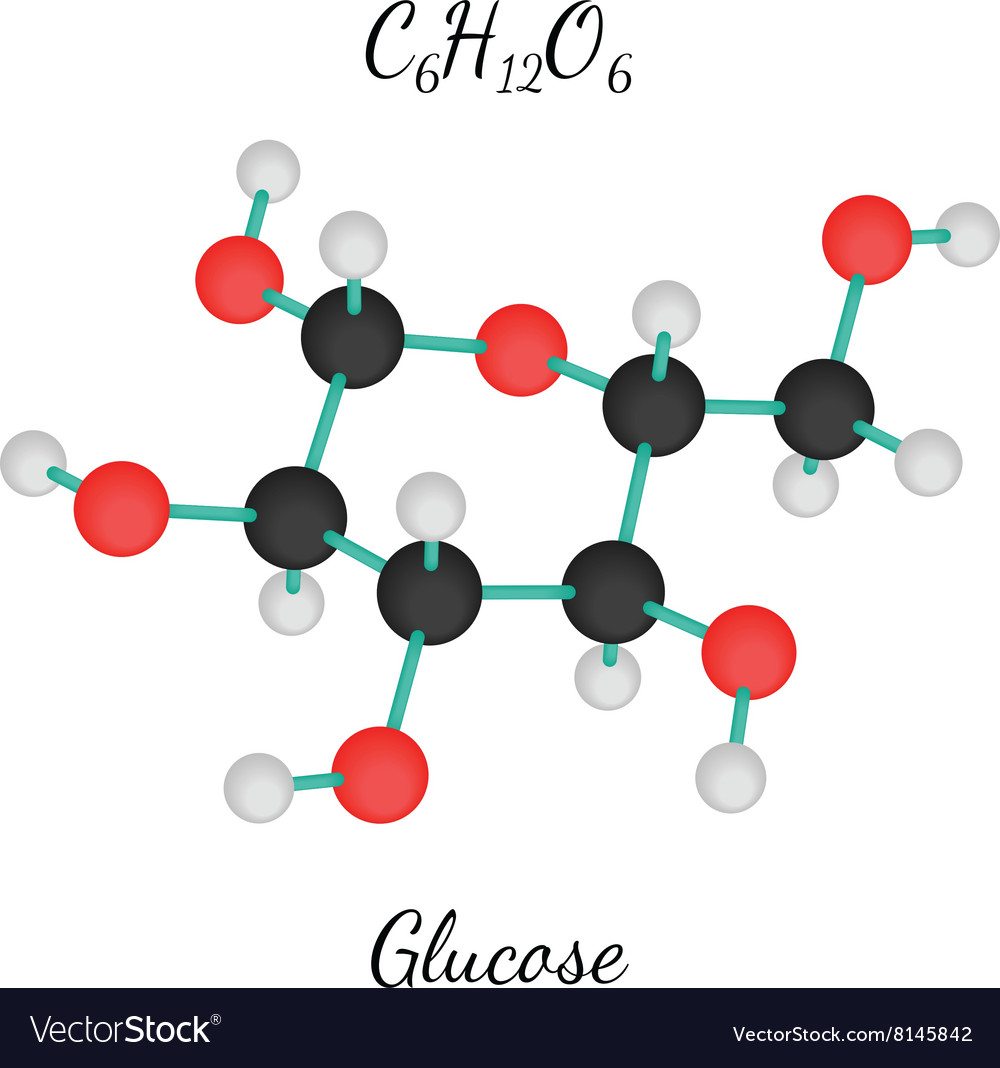
In humans, glucose is an important source of energy. The chemical formula for glucose is C 6H 12O 6. Trioses, pentoses, and hexoses have three, five, and six carbon backbones, respectively. Aldoses have a carbonyl group (indicated in green) at the end of the carbon chain, and ketoses have a carbonyl group in the middle of the carbon chain. Monosaccharides are classified based on the position of their carbonyl group and the number of carbons in the backbone.
See Figure 1 for an illustration of the monosaccharides. Depending on the number of carbons in the sugar, they also may be known as trioses (three carbons), pentoses (five carbons), and or hexoses (six carbons).
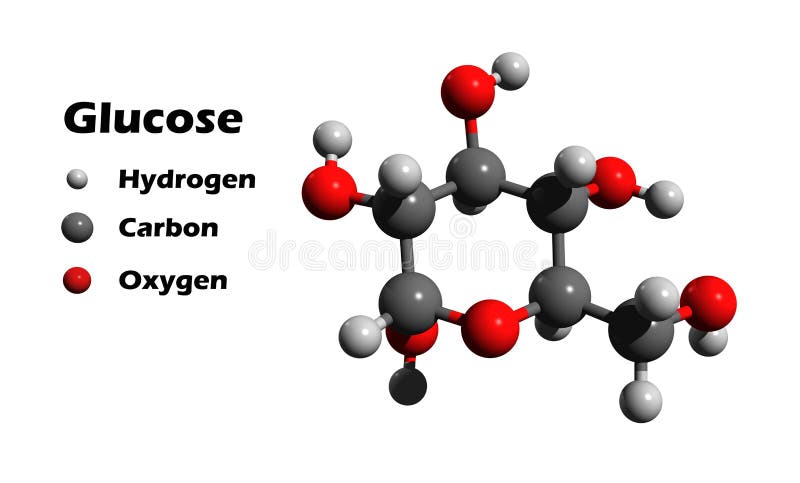
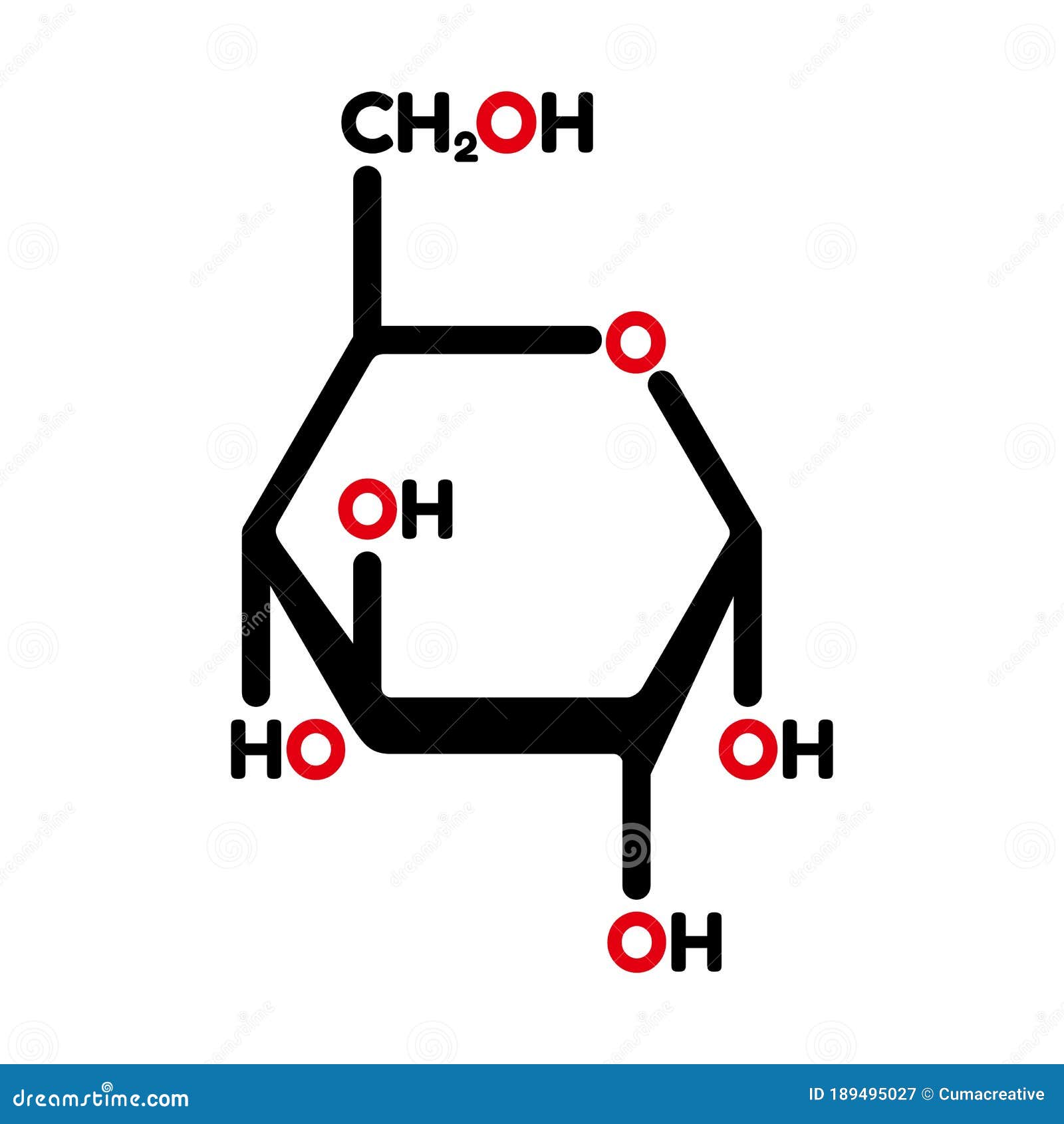
If the sugar has an aldehyde group (the functional group with the structure R-CHO), it is known as an aldose, and if it has a ketone group (the functional group with the structure RC(=O)R′), it is known as a ketose. Most monosaccharide names end with the suffix – ose. In monosaccharides, the number of carbons usually ranges from three to seven. Monosaccharides ( mono– = “one” sacchar– = “sweet”) are simple sugars, the most common of which is glucose. See the carbohydrates section of the 3-D molecules index, above.\) Glucose is a component of other biological molecules: maltose, lactose, sucrose, cellobiose, amylose, amylopectin, glycogen. It is in equilibrium with a open-chain form in which Carbon 1 forms a CHO aldehyde group which gives it reducing properties, so that it reacts with reagents such as Benedict's. The glucose molecule in this form is known as α-D-glucopyranose, as the central part of the molecule is similar to pyran - a six-membered heterocyclic ring-shaped compound with five carbon atoms and one oxygen. Only the carbon outside the ring (number 6) has 2 single hydrogens and an OH group. The ring itself is 6-sided, but only 5 of its corners are made up by carbon atoms. The glucose molecule can form into other configurations, but this structure - a ring or chair form - is the most stable and therefore most common in biological systems. The hydrogen atoms (white) are either attached directly to the carbons, or via oxygen as OH groups - at an angle. Notice the 6 carbon atoms (grey) forming the backbone of the molecule, and the oxygen atom (red) in the ring. It is an example of a 6-carbon ( hexose) sugar. Is a monosaccharide - formula C 6H 12O 6. Glucose (also known as dextrose or blood sugar)


 0 kommentar(er)
0 kommentar(er)
